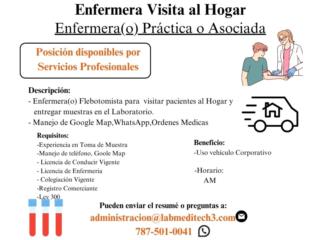
|
DescripciónDescription
The incumbent will support the Packaging Requalification Program, in conformance with ISO 11607 for all SKUs. Activities will include: Data Gathering (Participate in the discovery and compilation of required documentation to be evaluated), Document Generation (Generate all documentation required, included but not limited to Change Control, Validation Protocols, Drawings, Routers, etc.) Validation Executions (Validation activities-IQ, OQ, PQ, Shipping and Shelf Life, TMV etc.). The incumbent will be working with cross functional areas on activities described above. Responsible for the delivery of tasks to ensure conformance to the project plan schedule. Will manage documents within electronic PLM system and use statistical analysis tools, such as Minitab. |
Requisitos Requirements
Bachelor’s Degree in Engineering or Sciences, with 5-7 years of manufacturing experience in regulated industries. Exposure to Packaging Validations, Design Control and Product/Process Transfer. Knowledge of ISO 11607 regulations for sterile barrier packaging, understanding of packaging and transit (transport) testing and performance test methods (conditioning, compression testing, drop testing, vibration testing, bubble leak test), knowledge in shelf-life validation (aging studies), sterile barrier sealing process validation and packaging validation, working experience in package and sealing integrity tests and package seal strength tests (seal strength (peel) test, burst test, creep test, bubble leak test and dye penetration).. Excellent communication skills in Spanish and English. Available to work extended hours in a day and weekends as required. Temporary contract (one year 251465)
|