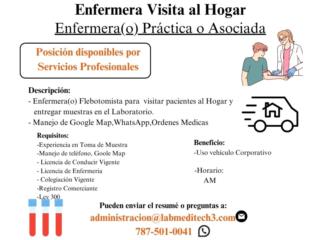
|
DescripciónDescription
Fundamental understanding of quality systems with implementation to domestic and international standards. Basic understanding of audit process: types of audits, planning, preparation, execution, reporting results, follow-up. Develop and implement quality programs: tracking, analyzing, reporting and problem solving. Plan, control and assure product and process quality in accordance with quality principles: planning processes, material control, acceptance sampling and measurement systems. Acquire and analyze data using appropriate standard quantitative methods across a spectrum of business environments to facilitate process analysis and improvements. Develop technical documentation: Validation Protocols, Technical/investigation reports, SOP, Validation Plans, Change Controls. Implement Quality System Regulation, ISO, Design Control and Process with strong Project Management skills. |
Requisitos Requirements
BS in Chem, Biomedical, Mech, or Ind. Eng, 5-7 years of experience in FDA regulated Medical devices environment. ASQ Certified Quality Engineer and/or Quality Audits, preferred. Validation guidelines and lyophilization preferred. Must have prior experience with ISO cleanrooms. Knowledge of qualifying new facility additions-HVAC, water filter systems. Knowledge in Lean and Six Sigma concepts and statistical techniques: SPC, DOE, Process Capability and Gage R & R, etc. with use of Minitab. Experience in biomaterials preferred. Working knowledge of business software (including MS Project, VISIO). Fully bilingual (English/Spanish). In-depth understanding of problem-solving and quality improvement tools and techniques. Basic knowledge of reliability, maintainability, and risk management, including key terms and definitions, assessment tools and reporting. Temporary contract (one yr. 251599)
|